Biophysical Laboratory
About the Laboratory
Biophysical Techniques in the Lab
Circular dichroism (CD) is a spectroscopic technique that measures the difference in the absorption (ΔA) of left-handed (AL) and right-handed (AR) polarized light which
CD = ΔA = AL – AR = (εL-εR)bc
Where ε is the molar absorptivity of left-handed or right-handed polarized light, b is the optical
Typical applications of CD include:
Protein folding – evaluation of secondary structure, tertiary structure
Conformation stability – evaluation of proteins stability by thermal, pH, and chemical (urea, guanidine HCl) stability
Quality control in the laboratory – Does protein structure change from batch-to-batch? Does storage change conformation? Different solution effects (buffers, salts, detergents, etc.) on the
Kinetic –
Protein-Protein Interactions
Instrument –Pistar-180 CD spectrometer
The Applied Photophysics
|
Light-Source: |
75W Xe Lamp (75W HgXe Lamp available for kinetics) |
|
Wavelength Range: |
180 - 850 nm using MgF2 optics |
|
Temperature Range: |
5 - 95 °C (external circulator) |
|
Kinetics: |
Millisecond deadtime (measure rate constants up to 1500 s-1). 1:1 and 1:10 volume ratio injections are available |
Dynamic Light Scattering (DLS), sometimes referred to as photon-correlation spectroscopy (PCS) or Quasi-Elastic Light Scattering (QELS), is a well-established technique that measures particle size distribution in the
Typical particles measured by DLS include:
- Protein characterization, including aggregation, conformation, structural and thermal stability
- Macromolecular complexes
- Surfactant and micellular systems
- Nanoparticles and colloidal dispersions
- Biological and synthetic polymer characterization
Instrument – DynaPro MS800
The SSSC has
Instrument Specifications
|
Size Range |
0.5 to 50 nm hydrodynamic radius |
|
Laser Diode |
830 nm (power adjustable) |
|
Temperature Range |
4 to 60 oC |
|
Sensitivity |
0.1 mg mL-1 for 14 kDa protein at 20 oC |
|
Cuvette |
Hellma 105.252-QS (b=1.5 mm, z=15mm, V=12 mL) |
Isothermal titration calorimetry (ITC) is a biophysical technique that allows the thermodynamic study of two interacting species. When these two species interact, heat is either generated or absorbed. By measuring these interaction heats, binding constants (K), reaction stoichiometry (n), and thermodynamic parameters including enthalpy (DH) and entropy (DS) can be accurately determined. In addition, varying the temperature of the experiment allows the determination of the heat capacity (DCp) for the reaction. Thermodynamic data
The basic principles of an ITC experiment are summarized in this Malvern short video. Some of the more common applications for ITC include biomolecular interactions (proteins, peptides, lipids, polysaccharides, small molecules, etc.). Other applications include surfactant characterization (
Instrument – Calorimetry Sciences 4200 Isothermal Titration Calorimeter
The SSSC is equipped with a Calorimetry Sciences Corporation (now part of TA Instruments) ITC4200 microcalorimeter which is equipped with removable Hastelloy cell assemblies. The CSC ITC allows researchers to study almost any kind of interaction, including solutes with immobilized enzymes, tissue samples, or other solid materials in suspension.
Typical experiments take approximately 30 min to stabilize the
Instrument Specifications
|
Affinity Constant (Ka) |
102 to 109 M-1 |
|
Competitive Binding Affinity Constant (Ka) |
109 to 1012 M-1 |
|
Minimum Detectable Heat |
0.1 mcal (0.4 mJ) |
|
Baseline Stability |
± 0.2 mcal s-1 hr (±0.08 mW hr-1) |
|
Cell Volume |
0.5-1.3 mL (full cells are recommended) |
|
Precision Buret |
25-250 mL |
|
Volume Increment |
1-20 mL (± 0.01 mL delivery precsion) |
|
Stir Rate |
0-500 rpm |
|
Temperature Range |
0-100 oC (Bath stability ±0.0005 oC at 25 oC) |
Surface Plasmon Resonance (SPR) measurements
The common advantages of this technique include being a label-free technique, real-time biomolecular interactions, and the relatively small sample volume requirements. In addition, there are many different sensor chips available to immobilize a variety of tagged biomolecules (His-tags, biotinylated, etc.). However, the kinetics of an interaction can provide insight towards the biomolecular interaction.
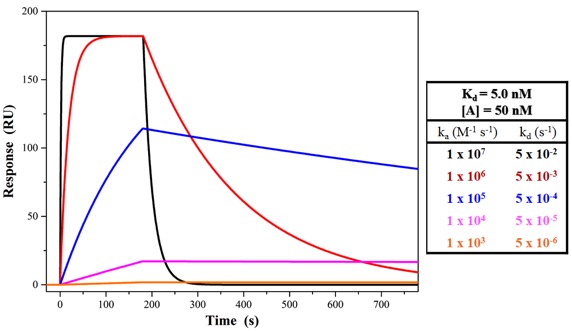
Why are biomolecular interaction kinetics important? Isn’t a KD good enough?
|
The affinity constant, KD, is generally used to assess the strength of binding in a biochemical interaction. However, the kinetics of the interaction can reveal significant and meaningful information that cannot be obtained by other methods that evaluate only KD. Interactions with the same affinity can have different orders of magnitude association and dissociation rate constants (see Figure 1). For example, SPR kinetic measurements are used extensively in the pharmaceutical industry in order to evaluate potential drug candidates. An antibody or small molecule that may have a high affinity (low KD) but a high |
Figure 1. SPR sensorgrams of showing the changes in binding kinetics (ka, |
Instrument –
The Biorad
Instrument Specifications
|
Refractive Index Range |
1.33 to 1.37 |
|
Number of Flow Channels |
6 Horizontal, 6 Vertical |
|
Number of Interaction Spots |
36 (0.2025 mm2) |
|
Number of Interspot References |
42 |
|
Detection Temperature Range |
15-40 oC (max. 10 oC below ambient temperature) |
|
Baseline Noise |
< 1 RU (< 20 kRu); < 1.5 RU (20-40 kRU) |
|
Baseline Drift |
< 1 RU/min (15-40 oC) |
|
Flow Rate |
25-200 µL/min |
|
Sample Injection Volume |
25-449 µL |
|
Minimum Sample Volume |
93 µL (35+25+8 uL for system+vial dead volume) |
|
AutoSampler |
2 96-well plates (8 x 12 configuration) |
|
Buffer Valves |
Switch between 2 different running buffers |
|
Online Degasser |
For Buffer system only |
|
Detection Limits (Typical Values*) |
|
|
Concentration |
10-3 to 10-10 M |
|
Association Rate Constant (ka) |
103 to 107 M-1 s-1 |
|
Dissociation Rate Constant (kd) |
10-5 to 10-1 s-1 |
|
Affinity (kinetic) (ka/kd) |
104 to 1011 M-1 |
|
Affinity (steady state) |
104 to 109 M-1 |
|
MW Limit* |
201 Da |
|
*Many experimental factors can expand or contract these working ranges and limits. Typical values achieved are listed. |
|
Training Information
The CD spectrometer is open to any academic research group. SSSC personnel will first train researchers on the instrument and offer assistance in experimental development if needed. Training typically lasts a few hours and SSSC personnel will typically assist researchers with measuring/optimizing their first measurements. After training, researchers may use the instrument independently and reserve the instrument time through SSSC Evolution site.
CD quality cuvettes (variable pathlengths) are available for general use, and small biophysical equipment available at the SSSC (pH meter, small centrifuge, etc.). Users are responsible for supplying their own reagents and consumables (pipette tips, Eppendorf tubes, etc.). Temporary storage of materials at the SSSC may be done with consent, but the SSSC is not responsible for any loss or damage to the materials stored at the SSSC.
The DLS instrument is open to any academic research group. SSSC personnel will first train researchers on the instrument and offer assistance in experimental development if needed. Training typically lasts 1-2 hr running a standard sample(s). Specific sample preparation and handling, as well as proper cuvette
The ITC instrument is open to any academic research group. SSSC personnel will first train researchers on the instrument and offer assistance in experimental development if needed. Training typically lasts about ½ day running a standard interaction, and the finer details on baseline stability and multiple sample handling will be discussed. SSSC personnel will typically assist researchers
The SPR instrument is open to any academic research group. SSSC personnel will first train researchers on the instrument and offer assistance in experimental development if needed. Training typically lasts most of a day where we complete a standard SPR interaction (pH-scouting, ligand immobilization, analyte interaction). SSSC personnel will typically assist researchers
Data Analysis Resources
- CDNN (
program is available at SSSC, only compatible up to Win7-32bit) - K2D2 (a copy of
program is available at SSSC) - DICROPROT
DICROWEB (registration for an account is required)- K2D3
- CAPITO
BESTSEL - Particle Sizing Systems (PSS) Webinar
- Horiba DLS Interpretation Webinar